21st Austria weekly - Marinomed (24/04/2019)
28.04.2019, 1076 Zeichen
Marinomed: Stock quoted biotech company Marinomed Biotech AG has announced the successful completion of the pivotal Phase III study for Budesolv. The top line results are now available and show that Budesolv achieves at least the same effect as the product which is currently on the market, with a significantly lower dose. The planned primary endpoint of the study for the first product of the innovative Marinosolv® technology platform has thus been achieved. The approval process can be continued as scheduled. As announced, the complete Phase III study with detailed results is expected and will be published by the end of the second quarter of 2019 at the latest.Marinomed has succeeded in increasing the bioavailability of hardly soluble compounds to treat sensitive tissues such as nose and eyes via the Marinosolv® technology platform. The platform's flagship product is Budesolv, a nasal spray for the treatment of allergic rhinitis.
Marinomed Biotech: weekly performance:
(From the 21st Austria weekly https://www.boerse-social.com/21staustria (24/04/2019)

Börsepeople im Podcast S16/05: Maximilian Lahrmann
Bildnachweis
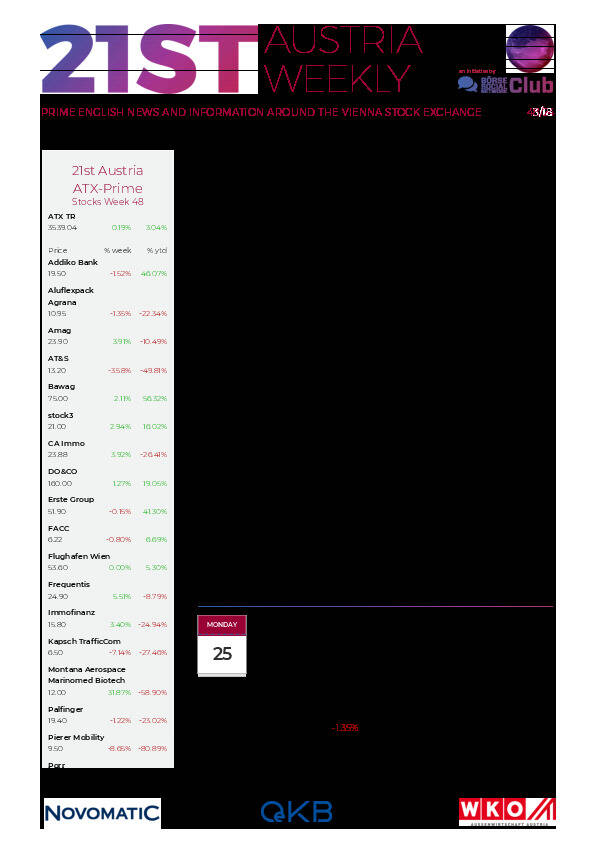
Aktien auf dem Radar:Pierer Mobility, voestalpine, Amag, Immofinanz, CA Immo, EuroTeleSites AG, Frequentis, Rosgix, Warimpex, Wienerberger, Kapsch TrafficCom, AT&S, Frauenthal, Gurktaler AG Stamm, Polytec Group, Wolftank-Adisa, Porr, Oberbank AG Stamm, UBM, Palfinger, Zumtobel, Addiko Bank, Agrana, Erste Group, EVN, Flughafen Wien, OMV, Österreichische Post, S Immo, Telekom Austria, Uniqa.
Random Partner
Cleen Energy AG
Die Cleen Energy AG ist im Bereich nachhaltige Stromerzeugung durch Photovoltaik-Anlagen und energieeffiziente LED-Lichtlösungen für Gemeinden, Gewerbe und Industrie, einem wichtigen internationalen Zukunfts- und Wachstumsmarkt, tätig.
Ein Fokusbereich ist das Umrüsten auf nachhaltige Gesamtlösungen. Zusätzlich baut CLEEN Energy den Bereich Leasing und Contracting von Licht- und Photovoltaikanlagen aus, der einen wachsenden Anteil am Umsatz ausmacht.
>> Besuchen Sie 68 weitere Partner auf boerse-social.com/partner
Useletter
Die Useletter "Morning Xpresso" und "Evening Xtrakt" heben sich deutlich von den gängigen Newslettern ab.
Beispiele ansehen bzw. kostenfrei anmelden. Wichtige Börse-Infos garantiert.
Newsletter abonnieren
Runplugged
Infos über neue Financial Literacy Audio Files für die Runplugged App
(kostenfrei downloaden über http://runplugged.com/spreadit)
per Newsletter erhalten