21st Austria weekly - Valneva (13/05/2024)
19.05.2024, 1068 Zeichen
Valneva: Valneva, a specialty vaccine company, today reported further positive pivotal Phase 3 data in adolescents for its single-shot chikungunya virus (CHIKV) vaccine. Following the initial analysis up to Day 29 post-vaccination, the most recent analysis of study VLA1553-321 evaluated the safety and immunogenicity six months (Day 180) after vaccination with a single dose of the vaccine. The Day 180 results confirm the initial positive immunogenicity and safety data Valneva reported previously, and are intended to support filing for potential label extension for use in adolescents aged 12 to 17 years. The data are also expected to support licensure of IXCHIQ® in Brazil, which would be the first potential approval for use in endemic populations. Three marketing applications are currently under review by the European Medicines Agency, Health Canada and the Brazilian Health Regulatory Agency (ANVISA) with potential approvals in 2024.
Valneva: weekly performance:
(From the 21st Austria weekly https://www.boerse-social.com/21staustria (13/05/2024)

SportWoche Podcast #140: Flag Football und Österreichs Top-Position dabei, erklärt von Ines-Jeanne Paupie
Bildnachweis
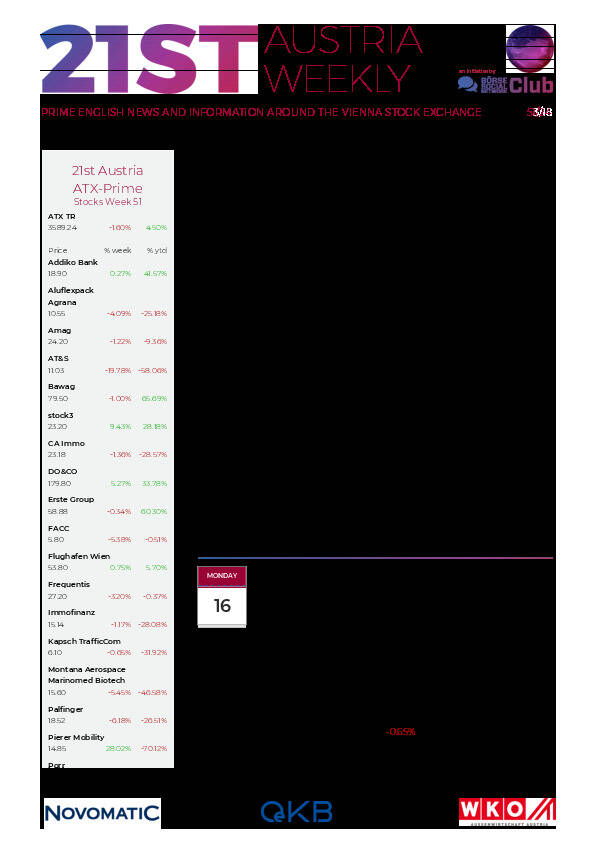
Aktien auf dem Radar:Pierer Mobility, UBM, Palfinger, Immofinanz, Addiko Bank, CA Immo, SBO, Porr, Rosenbauer, EuroTeleSites AG, Frequentis, Kostad, Linz Textil Holding, Marinomed Biotech, Wiener Privatbank, Warimpex, Agrana, Amag, EVN, Flughafen Wien, OMV, Österreichische Post, Telekom Austria, Uniqa, VIG, E.ON , BASF, Zalando, Mercedes-Benz Group, Allianz, Hannover Rück.
Random Partner
FACC
Die FACC ist führend in der Entwicklung und Produktion von Komponenten und Systemen aus Composite-Materialien. Die FACC Leichtbaulösungen sorgen in Verkehrs-, Fracht-, Businessflugzeugen und Hubschraubern für Sicherheit und Gewichtsersparnis, aber auch Schallreduktion. Zu den Kunden zählen u.a. wichtige Flugzeug- und Triebwerkshersteller.
>> Besuchen Sie 68 weitere Partner auf boerse-social.com/partner
Useletter
Die Useletter "Morning Xpresso" und "Evening Xtrakt" heben sich deutlich von den gängigen Newslettern ab.
Beispiele ansehen bzw. kostenfrei anmelden. Wichtige Börse-Infos garantiert.
Newsletter abonnieren
Runplugged
Infos über neue Financial Literacy Audio Files für die Runplugged App
(kostenfrei downloaden über http://runplugged.com/spreadit)
per Newsletter erhalten